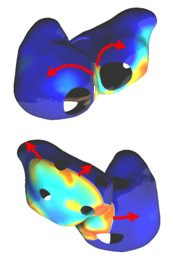
|
|
Atrial fibrillation (AF, Afib) is the most frequent arrhythmia
in clinical practice and a major cause of morbidity and mortality. A recent study estimated that the number of Americans afflicted
by AF will increase from the current range of 2.2 to 5.6 million to more than 12 million by 2050. It occurs globally. The
enormity of the clinical problem is magnified by well-described sequelae: thromboembolic stroke, congestive heart failure,
increased mortality and cognitive dysfunction. AF costs Medicare more than 15.7 billion annually due to costly complications.
Current AF therapies, including anti-coagulation drugs, anti-arrhythmia drugs, and catheter ablation, are frequently inadequate
and problematic. Unlike for ventricular fibrillation, there are no implantable atrial cardioverters (IACs) on the market.
A first generation IAC, developed by InControl, was trialed in patients in the early 1990s. It was shown to provide prompt
and safe restoration of sinus rhythm in patients with recurrent tachyarrhythmias. However, it was not introduced to the market,
primarily because patients could not tolerate the discomfort caused by the shocks, which required approximately 3 Joules using
conventional biphasic waveform shocks. Although contemporary defibrillators or CRD devices might include atrial therapy features,
these functions are often turned off. Recently, there have been many advances in radio-frequency catheter ablation technology,
however, such long procedures could lead to more complications. Also, AF episodes often recur, which requires more ablation
procedures. Thus, special education regarding the treatment choice is warranted for patients. Current
AF clinical management is challenging; it presents a unique opportunity to engineers and scientists to develop collaborative
innovations. As a newly established research and consulting firm, AFI (AF-innovation) LLC is comprised of key consultants,
including industry experienced and skilled scientists. The company will: (1) pursue state-of-art of catheter ablation technologies;
(2) provide computational 3D image electro-anatomical mapping modeling research tools for its clients; (3) provide clinical
education/training consultations for atrial fibrillation or other cardiac rhythm disorder patients. As an AF technology advocate, the company is located in the cluster of St. Louis biotechnology center: Center for Emerging Technologies/Cortex, which is just a few miles away from the Washington University Medical Center.  AF Innovation LLC. 4041
Forest Park Avenue St. Louis, MO 63108 Phone: (314) 615 6900 (CET) Email: contact@af-innovation.com
|